The driver mutations causing Hematopoietic Stem Cell (HSC) expansion in Clonal Hematopoiesis (CH) are inherited by HSC progeny and in some settings alter the function of mutant immune cells. It is unclear how CH mutations impact immune cell function in response to immune checkpoint blockade (ICB), which is a critical cancer therapy for many solid tumours and hematological cancers. Understanding how CH influences ICB could provide a novel biomarker for patient stratification or personalized therapeutics and may offer insight into immunotherapy mechanisms, yielding novel drug targets to synergistically improve patient outcomes.
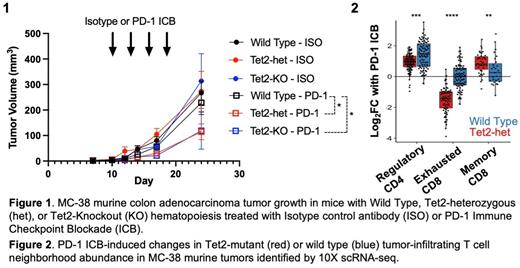
Somatic mutations inactivating TET2, which is responsible for 5-methylcytosine hydroxylation and demethylation, are one of the most frequent drivers of CH. TET2 inactivation can enhance inflammation from monocytes/macrophages, CD4 + and CD8 + T cells, but its impact on ICB is unknown. Here we sought to define the impact of TET2-mutant CH (TET2-CH) on ICB response. First, we modelled TET2-CH by rescuing lethally irradiated C57BL6/j mice with Tet2-knockout (-KO), Tet2-heterozygous null (-het), or wild type (WT) bone marrow. Mice were subsequently implanted with the syngeneic colorectal adenocarcinoma line MC38 and treated with Programmed Cell Death Protein-1 (PD-1) targeted ICB or Isotype Control (control) antibodies. PD-1 ICB, but not control, treated tumors were significantly smaller in mice with Tet2-het or Tet2-KO versus WT hematopoiesis (Figure 1). There was a trend towards a dose-responsive effect, with more Tet2-het mice responding to PD-1 ICB than wt, and more Tet2-KO mice responding than Tet2-het or wt mice. Therefore, Tet2-mutant leukocytes promote response to PD-1 blockade. PD-1 ICB with targeted cell depletion revealed that the enhanced response in Tet2-het mice requires CD4 + T Cells, CD8 + T Cells and macrophages, but not NK cells.
To understand the mechanism through which Tet2-inactivation promotes ICB responses we performed 10X single cell RNA-sequencing on 38,994 CD45 + tumor-infiltrating leukocytes (TILs) from Tet2-het and wt mice treated with PD-1 ICB or control. Tet2-het mutations dramatically changed the TIL landscape with PD-1 blockade by promoting the acquisition of cell fates that drive durable immunotherapy responses including CD8 + effector T cells, CD8 + memory T cells, CD4 + interferon-responsive cells, and macrophages expressing an inflammatory anti-tumour gene signature (Figure 2). In contrast, wt TILs from PD-1 treated tumours were significantly enriched for exhausted CD8 + T cells, CD4 + regulatory T cells, and immunosuppressive macrophages that can promote tumor growth (Figure 2). TIL DNA methylation analysis using Infinium Beadchip arrays revealed promoter hypermethylation in genes defining the immunosuppressive macrophage state. Collectively, this suggests that Tet2-CH reshapes TIL fate following ICB by promoting anti-tumor phenotypes and protecting cells from immunosuppressive states.
To investigate the potential clinical relevance of our findings, we retrospectively tested for an association between TET2-CH and immunotherapy outcome in 569 ICB-treated solid tumor patients. CH mutations were detected in publicly-available exome-sequencing data at VAF >0.02 using Mutect2. While CH as a composite of all driver mutations did not correlate with outcome, in multivariate analysis TET2-CH was associated with 6-times greater odds of achieving 6 month clinical benefit in n=205 melanoma patients (95% CI 1.01-66.6). To explore whether TET2-CH might identify patients primed for ICB response, we screened for CH in the prospectively collected baseline PBMCs from 90 solid tumour patients treated in a Phase 2 trial of the PD-1 inhibitor Pembrolizumab (NCT02644369). While underpowered to draw conclusions, we noted that one patient, treated for the skin cancer Merkel Cell Carcinoma, was positive for TET2-CH and responded to ICB, and also survived longer than the cohort median and Merkel Cell Carcinoma median.
In summary, our results show that TET2-CH promotes immunotherapy response by shifting TILs from immunosuppressive to anti-tumor fates. Mechanistically, this occurs through lineage-specific promoter hypermethylation and gene silencing. Further study of TET2-CH as a potential biomarker of ICB response is warranted and targeting TET2-regulated pathways may yield novel strategies to improve immunotherapy outcomes.
Disclosures
Chan:Agios: Research Funding; Servier: Research Funding; AbbVie: Research Funding; BMS: Research Funding. Dick:Trillium Therapeutics Inc/Pfizer: Patents & Royalties: Trillium Therapeutics; Celgene/BMS.: Research Funding; Graphite Bio: Consultancy, Membership on an entity's Board of Directors or advisory committees.